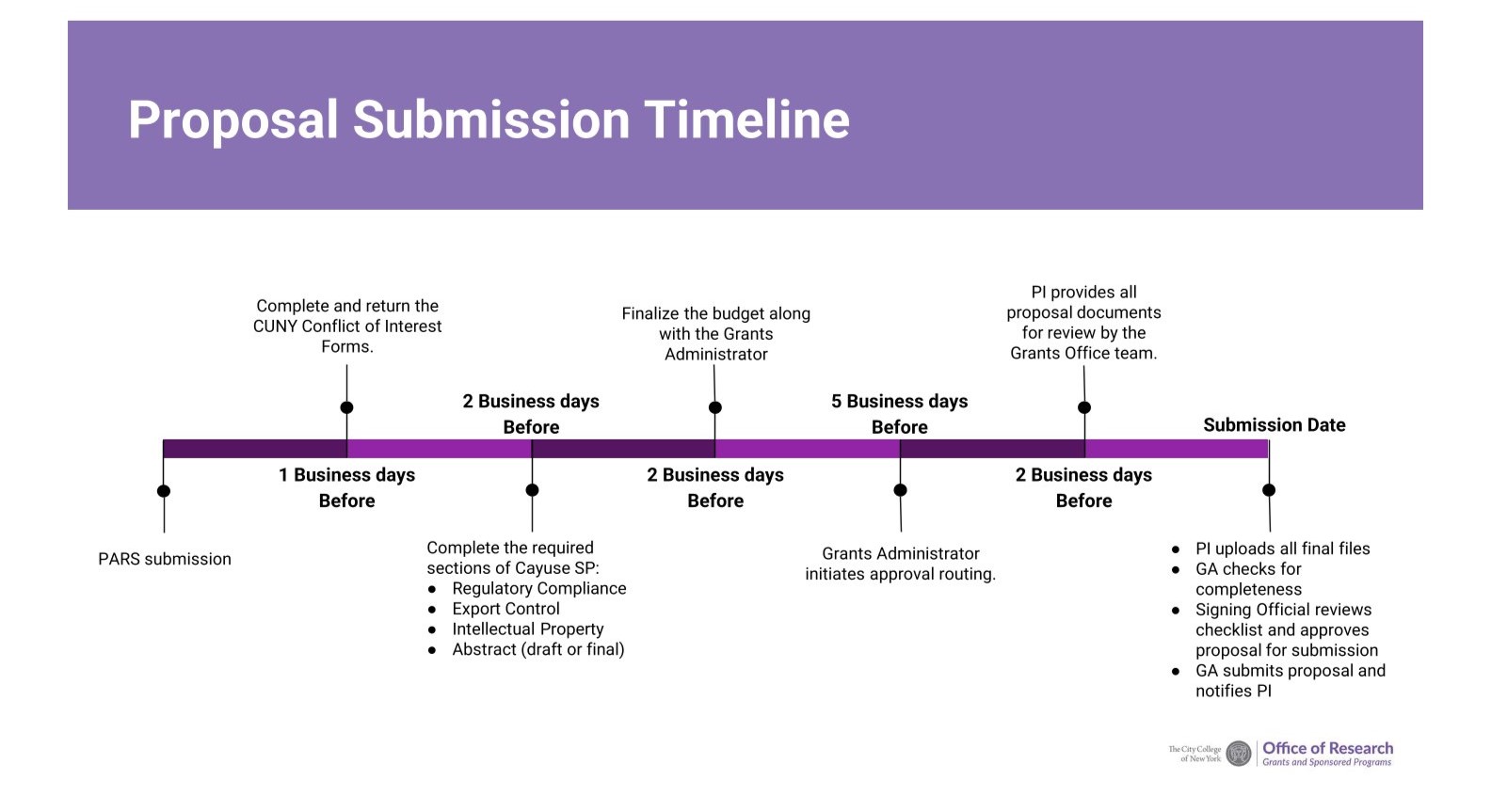
Grants and Sponsored Programs (GSP) Pre-award team assists faculty and other principal investigators (PIs) throughout the proposal preparation and submission process. In order to have sufficient time to review the sponsor's guidelines, develop accurate budgets, prepare institutional documents and ensure a fully compliant application, GSP has established the following internal deadlines:
10 business days (2 weeks) before the submission deadline
- PI notifies GSP of planned submission via our Proposal Assistance Request System - PARS To learn more, PARS page
- Sponsor guidelines
- Lead institution requirements (if subcontract to CCNY)
- Tentative budget categories and amounts
- GSP Grants Administrator creates proposal record in Cayuse SP (within 2 days of receiving PARS)
5 business days (1 week) before the submission deadline
- PI provides these proposal elements:
- Completed FCOI forms
- Completed RCR certificate (if not provided at time of PARS submission)
- Final budget categories and amounts
- Draft abstract
- Completed compliance questions in Cayuse SP
- Animal and Human Subjects
- Export Control
- Intellectual Property
- GSP initiates internal routing and approval in Cayuse SP
3 business days before the submission deadline
- All affiliated Chairs, Deans and other administrators approve proposal for submission
- % Effort of all investigators
- Cost sharing (allowed only if required by sponsor)
- Appropriateness of project
2 Business days before submission deadline
- All final documents, such as:
- Proposal narrative
- References
- Summary/Abstract
- Budget Justification
- CVs/bio sketches
- Current and pending support
- Facilities documents
- Postdoctoral mentoring plans
- Letters of commitment
1 business day before the submission deadline
- GSP submits the proposal to the sponsor
Day of submission deadline
- GSP checks for any submission errors and works with PI on corrections
- Resubmit proposal if necessary
Sponsor update
NSF Updates
The National Science Foundation (NSF) has announced the issuance of the revised Proposal and Award Policies and Procedures Guide (PAPPG) (NSF 24-1) effective for proposals submitted or due on or after May 20, 2024. A complete summary of changes can be found at: Summary of Changes.
Notable pre-award submission changes include:
- Biographical Sketches: the updated guidance serves as NSF’s implementation of the biographical sketch common form (SciENcv),
- The 3-page limit has been removed. There is no longer a page limit for this section of the proposal.
- New Synergistic Activities Document:
- The Synergistic Activities section has been removed from the biographical sketch. This information must now be submitted by individuals designated as senior/key persons as part of the senior/key personnel documents in Research.gov. The document must be no more than one-page and includes a list of up to five distinct examples that demonstrates the broader impact of the individual’s professional and scholarly activities that focus on the integration and transfer of knowledge as well as its creation.
- Current and Pending (Other) Support: the updated guidance serves as NSF’s implementation of the common form. The Current and Pending Support form will continue to be created in SciENcv, which will produce a pdf compliant version that can be attached to the proposal in Research.gov.
- Mentoring Plan: Mentoring plans are now required when either postdoctoral researchers OR graduate students are supported on an NSF proposal. The one-page plan must be loaded in the “Supplementary Documents” section. Full details of the requirement are located at: Mentoring Plan Requirement.
- Foreign Organizations: Proposers must justify any request that includes a foreign organization (subaward) or foreign individual (consultant) in the budget. A justification of the benefits to the research and education is required.
- Malign Foreign Talent Recruitment Certification: Certifications must be made on the biosketch, current and pending support, and other affiliation documents that each senior/key personnel is not party to a malign foreign talent recruitment program.
- Proposal Font, Spacing, and Margin Requirements: has been modified to allow for submission of proposal documents in landscape format.
NIH Updates
Significant Changes (This section details all significant changes and revisions made to the instructions since the last major release).
Noted Changes
Data Management and Sharing (DMS) Plans: DMS Plans are now included in Section 11. Other Plan(s). Plans for Genomic Data Sharing should be provided as part of the Data Management and Sharing Plan.
Refer to the list of NIH activity codes that requires DMS Policy or your Funding Opportunity Announcement to determine if your application is required to provide a Data Management and Sharing (DMS) Plan.
Data Management and Sharing Plan Format Page
- R&R Budget and associated R&R Subaward Budget Attachment(s) Form:
- Updated special instructions for applicants submitting a Data Management and Sharing (DMS) Plan within the following sections:
- If a Data Management and Sharing Plan is required in the proposed application costs to support these activities, may be requested in the appropriate cost category. Details regarding Data Management and Sharing costs must be specified in the Budget Justification attachment (L), pursuant to the instructions.
- Updated special instructions for applicants submitting a Data Management and Sharing (DMS) Plan within the following sections:
- Budget justification:
- If a Data Management and Sharing Plan is required in the proposed application, include a brief justification of the proposed activities that will incur costs. The Data Management and Sharing justification must be clearly labeled as "Data Management and Sharing Justification" within the budget justification attachment followed by the estimated dollar amount (total direct costs). Provide a brief summary of type and amount of scientific data to be preserved and shared and the name of the established repository(ies) where they will be preserved and shared. Indicate general cost categories such as curating data and developing supporting documentation, local data management activities, preserving and sharing data through established repositories, etc., including an amount for each category and a brief explanation. Specify in the justification if no costs will be incurred for Data Management and Sharing, if applicable. The recommended length of the justification should be no more than half a page.
Link to Sponsors General Guidelines:
- NSF PAPPG - 23-1 - Current version
- NSF PAPPG - 24-1 - Effective for proposals submitted or due on or after May 20, 2024
- NIH Forms H
- NASA Proposer's Guide
- ROSES-2024 Summary of Solicitation
Frequently used Templates
Common Forms template link
NIH
NSF
Cost Sharing Policy
Cost sharing is the portion of sponsored project costs (direct and indirect) not borne by the extramural sponsor; thus, the City College absorbs the cost. Compliance with federal cost accounting standards requires that Cost Shared expenses be treated in a consistent and uniform manner in proposal preparation, award negotiation and the accounting of these expenses in the financial reports to sponsors.
Any Cost Sharing included in the award budget is a condition of the award and is subject to audit.
There are two types of Cost Sharing which require both tracking and reporting:
- Mandatory Cost Sharing: Project costs that are not paid by the sponsor and are required as a condition of the award.
- Voluntary Committed Cost Sharing: Costs specifically pledged on a voluntary basis and specifically included in the award budget.
More info on cost sharing:
Last Updated: 05/15/2024 16:17