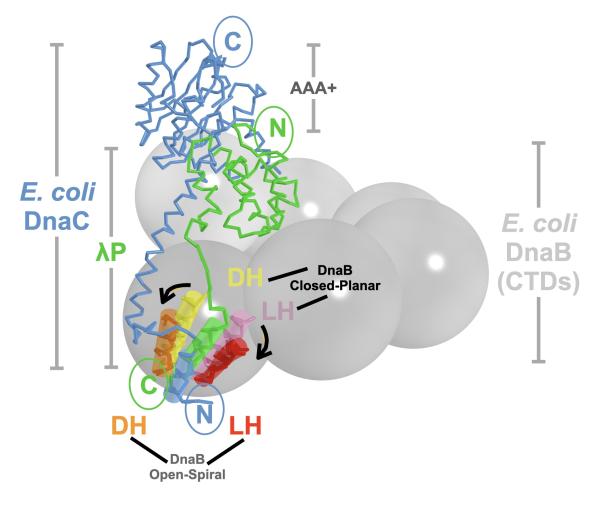
Two structurally unrelated bacterial helicase loaders (light blue: DnaC and green: λP) converged on the same helicase opening strategy by positioning a single alpha helix (blue and green transparent cylinders) in virtually the same spot on the helicase to open it.
DNA replication is the foundation of all life. During its first stage, an initiator protein binds to double-stranded DNA at an AT-rich site, where the base pairs are melted and the double helix separates into two single strands. A single DNA strand is shepherded into a hexameric ring shaped protein, or helicase, which operates as a molecular motor. The helicase relies on specialized proteins, helicase loaders, which effectively hook the helicase at points on the source DNA. One of their key roles in bacterial DNA replication is opening the helicase for DNA loading.
The bacterial helicase protein, DnaB, is the same across most bacteria. This is not true for the loaders. In E. coli and Phage λ, both DnaC and λP perform two essential loading functions: ring breaking, in which the DnaB hexamers are opened, and shepherding DNA into the opened ring. They are key for DNA replication in their respective organisms, and both bind to single-strand DNA.
City College of New York biologist David Jeruzalmi and colleagues studied the mechanism by which two genetically distinct helicase loaders evolved convergently to perform the same function.
Although these functions may point to a common origin, DnaC and λP are unrelated in DNA sequence and enzymatic functions. Rather than evolving from a common ancestor they converged over time because of a physical requirement: opening the DnaB helicase to permit the entrance of the DNA strand.
“Think of these proteins as both needing to open a door. If they didn’t have a common ancestor, they had to separately evolve ways to turn the handle,” said Jeruzalmi. “Because there is only one site on DnaB where the door can be opened, like a spring latch, both proteins are constrained to open the DnaB helicase in the same way,” he said.
The implications for this discovery are far reaching. Every bacterium uses the DnaB protein helicase structure during its life cycle to replicate. The loaders open and close the DnaB helicase as needed.
“If we developed a molecule that forces [the helicase] to stay open, the cell can’t replicate. That’s a new antibiotic,” said Jeruzalmi.
The study appears in the journal Trends in Biochemical Sciences.
About the City College of New York
Since 1847, The City College of New York has provided a high-quality and affordable education to generations of New Yorkers in a wide variety of disciplines. CCNY embraces its position at the forefront of social change. It is ranked #1 by the Harvard-based Opportunity Insights out of 369 selective public colleges in the United States on the overall mobility index. This measure reflects both access and outcomes, representing the likelihood that a student at CCNY can move up two or more income quintiles. In addition, the Center for World University Rankings places CCNY in the top 1.8% of universities worldwide in terms of academic excellence. Labor analytics firm Emsi puts at $1.9 billion CCNY’s annual economic impact on the regional economy (5 boroughs and 5 adjacent counties) and quantifies the “for dollar” return on investment to students, taxpayers and society. At City College, more than 16,000 students pursue undergraduate and graduate degrees in eight schools and divisions, driven by significant funded research, creativity and scholarship. CCNY is as diverse, dynamic and visionary as New York City itself. View CCNY Media Kit.
Erica Rex
p: 845 668 0322
e:
erex@ccny.cuny.edu