Christopher K. Chong
Mentor: Dr. Robert J. Messinger, CUNY Energy Institute
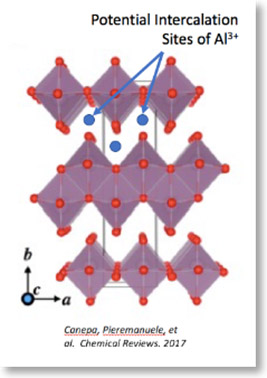
Lithium-ion batteries have revolutionized portable electronics. With an increase in the demand for more efficient and low-cost means of energy storage, particularly for the electric grid and for electric vehicles, there has been growing research interest in finding alternative battery technologies. Aluminum metal is the most commonly found metal on Earth’s crust, is low cost, non-toxic, and non-flammable. Aluminum also has the highest volumetric capacity long all common metal electrodes, almost four times greater than lithium metal. Aluminum battery technology is an emerging technology that has not been explored in comparison to other battery chemistries, including lithium, sodium, magnesium, and zinc batteries. Few cathode materials have been shown to reversibly electrochemically cycle with aluminum metal anodes. This research focuses on transition metal oxides as intercalation cathodes for rechargeable aluminum batteries, specifically α-MoO3 nanowires. α-MoO3 is an interesting material because of its predicted theoretical energy density of 3630 Wh/L and theoretical specific capacity of 470 mAh/g. The objective of this research is to hydrothermally synthesize α-MoO3 nanowires and perform electrochemical analysis on Al-MoO3 batteries to establish the feasibility of this new electrode pair and to understand its electrochemical properties and reaction mechanisms. The results shed light on the challenges and opportunities of emerging aluminum battery technologies.
Structure of α-MoO3 with potential intercalation sites of Al3+ ions
Last Updated: 08/29/2023 16:28