The Biological Safety Program, part of EHOS Office, manages and evaluates biological activities, waste disposal, and research across CCNY to ensure compliance with local, state, and federal regulations. To help protect the health and safety of researchers, students, and the environment, EHOS collaborates with the Institutional Biosafety Committee (IBC) and the Institutional Animal Care and Use Committee (IACUC) on issues related to the safe conduct of biological research and requires users of biological materials and pathogens to be trained and follow guidelines.
POLICIES AND PROCEDURES
BLOODBORNE PATHOGENS AND TRAINING
PIs and lab personnel working in a BSL-1 or BSL-2 environment are required to take annual bloodborne pathogen training, either through scheduled sessions with EHOS members or the CITI training service. Responsible individuals working in these locations should familiarize themselves with the annually reviewed CCNY Exposure Control Plan to be prepared in case of a biological emergency.
STANDARD OPERATING PROCEDURES
Standard Operating Procedure templates are available for processes involving biological materials in both BSL-1 and BSL-2 laboratories that differ from the template provided by EHOS for non-biological procedures. PIs working with biohazardous materials are recommended to contact EHOS when developing standard operating procedures to receive these templates when necessary. Any questions regarding the development of biological SOPs or the initiation of experiments that require IACUC clearance should be directed to the Institutional Animal Care and Use Committee.
FORMS AND FACT SHEETS
- Autoclaves and Sterilizers
- Biohazard Supplies - Contact 212-650-5080 and leave a message with room location, PI, and quantity of boxes, bags, sharps containers and biohazard tape.
- Institutional Biosafety Committee (IBC) and Research Involving Recombinant or Synthetic Nucleic Acid Molecules – Contact ehos@ccny.cuny.edu or at 212-650-5080 for information about the IBC application process.
FREQUENTLY ASKED QUESTIONS ABOUT BLOOD BORNE PATHOGENS
Q. What is a blood borne pathogens?
A. Microorganisms, like viruses or bacteria, that are present in human blood and can cause disease in humans, including Hepatitis B, Hepatitis C and HIV.
Q. What are routes of entry?
A. direct contact with infected blood or body fluids, specifically via broken skin (like needlesticks, cuts, or abrasions) or mucous membranes (eyes, nose, mouth).
Q. What is considered an exposure to biological materials?
A. According to OSHA coming in direct contact with the eye, mouth, other mucous membrane, non-intact skin, or contact with blood or other potentially infectious materials (OPIM) that results from the performance of an employee's duties.
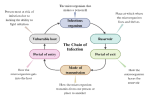
Q. What is the infection cycle? How is it broken?
A. The chain is broken through hand washing PPE and in some cases vaccination vaccines
Q. What is universal precaution?
A. Universal precaution is where you treat everything as if its infectious also wear personal protective equipment PPE such as gloves lab coat and goggles as needed and hand washing.
Q. How to clean down bench tops when working with biological infectious materials?
A. Chemical hygiene plan you can use 70% ethanol or 10% bleach to wipe down bench tops.
Q. How does hand washing help prevent infections?
A. Washing your hands with both soap and water breaks up both soaps break open envelope viruses and water breakdown non envelope viruses.
Q. How to clean up a minor biological spill?
A. Chemical hygiene plan
- Biological spills outside biological safety cabinets can pose the danger of aerosol generation and dispersion. These spills can be serious.
- To reduce the risk of inhalation exposure in such an incident, occupants should leave the laboratory immediately.
- The laboratory should not be reentered to decontaminate and clean up the spill for at least 30 minutes. During this time the aerosol should be removed from the laboratory by the exhaust air ventilation system.
Spill Involving a Microorganism Requiring BL 1 containment
- Wear disposable gloves
- Soak paper towels in disinfectant and place over spill area
- Place towels in plastic bag for disposal
- Clean spill area with fresh towels soaked in disinfectant.
Spill Involving a Microorganism Requiring BL 2 containment
- Alert people in immediate area of spill
- Put on protective equipment
- Cover spill with paper towels or other absorbent materials
- Carefully pour a freshly prepared 1 in 10 dilution of household bleach around the edges of the spill and then into the spill. Avoid splashing.
- Allow a 20-minute contact period
- Use paper towels to wipe up the spill, working from the edges into the center
- Clean spill area with fresh towels soaked in disinfectant
- Place towels in a plastic bag and decontaminate in an autoclave.
Biosafety
Q. What is biosafety?
A. Biosafety is focused on protecting individuals, agriculture, and the environment from potentially harmful microorganisms and other biological agents through risk assessment, work practices, protective equipment, and exposure control.
Q. What is the IBC?
A. The IBC stands for the Institutional Bio safety committee. The responsibility of the committee is to review protocols that involve the use of recombinant DNA or synthetic nucleic acids. Before the laboratory starts conducting that research to ensure that the it is conducted safely.
Q. What comprises an IBC committee?
A. The IBC committee is comprised of at least 5 members but it can be much larger based on need number of protocols that the committee reviews. If there is a large volume of protocols that the committee reviews then the number of members could be large.
Q. How many members are needed to form an IBC committee?
A. According to National Institute of Health (NIH) guidelines you need at least 5 members on the committee to have an Institutional Biosafety Committee.
Q. What is a quorum?
A. To have an IBC meeting you would need at least 50% of the number of voting members to hold a meeting.
Q. What types of protocols are typically reviewed by the IBC committee?
A. Protocols that involve the use of recombinant DNA or synthetic nucleic acids or biohazardous materials would be reviewed by the IBC committee.
Q. What types of protocols are exempt from review by the IBC?
- Protocols that fall under section III-F of the NIH guidelines
- DNA segments from a single non chromosomal or viral DNA source or from a prokaryotic host.
- Or from a bacterium that has a plasmid associated with it or virus when replicated only in that host.
- Synthetic Nucleic acid that is not designed to replicate or integrate into DNA and do not produce toxins LD50 or less than 100 nano grams per kilograms body weight are exempt.
- Yeast and E. coli K-12
- Test tube PCR reactions that do not involve cloning and propagation of recombinant DNA in cells.
- Commercially available de regulated transgenic crop.
Q. What status are given to protocols after review IBC review?
A. When the IBC committee is completed reviewing the protocols they are given
Full board approval means that the protocol has been fully approved by the committee and research can begin once they get the letter which is usually sent out electronically.
Conditional approval conditional approval means that the committee has reviewed the protocol but there are conditions that needs to be satisfied by the committee before the lab can start doing the research. This could be more clarity is needed, diagrams need to be fixed additional safety steps needs to be added to the protocol. The protocol is then sent back to the committee to se if the revisions were made and if the committee is satisfied with the revisions, it is then given full board approval.
Tabled means that the protocol will be discussed at a later time nor information is needed, may need guidance for additional sources, major information might be missing so the committee is not able to do a full risk assessment based on what they currently have.
Q. When is a biosafety officer needed?
A. A biosafety officer is needed when there are laboratories at the levels above BSL-2, Such as
BSL-3 and BSL-4. Then a biosafety office is required under the NIH guidelines.
Last Updated: 05/30/2025 12:24